Retrospective Chart Review of Prostate Cancer Workup in Compliance With Institutional Guidelines
Abstruse
To report outcomes and risk factors of ultrahypofractionated (UHF) radiotherapy for Japanese prostate cancer patients. This multi-institutional retrospective analysis comprised 259 patients with localized prostate cancer from 6 hospitals. A total dose of 35–36 Gy in iv–v fractions was prescribed for sequential or alternate-solar day assistants. Biochemical failure was divers according to the Phoenix ASTRO consensus. Toxicities were assessed using National Cancer Institute Common Toxicity Criteria version iv. Tumor control and toxicity rates were analyzed by competing hazard frames. Median follow-upward duration was 32 months (range 22–97 months). ii- and 3-twelvemonth biochemical control rates were 97.7% and 96.iv%, respectively. Initial prostate-specific antigen (p < 0.01) and neoadjuvant androgen deprivation therapy (p < 0.05) were identified as take chances factors for biochemical recurrence. two- and three-year cumulative ≥ Grade 2 belatedly genitourinary (GU) toxicities were v.8% and 7.4%, respectively. Corresponding rates of gastrointestinal (GI) toxicities were 3.nine% and iv.5%, respectively. Grade 3 rates were lower than 1% for both GU and GI toxicities. No grade 4 or college toxicities were encountered. Biologically effective dose was identified every bit a adventure factor for ≥ Form 2 belatedly GU and GI toxicities (p < 0.05). UHF radiotherapy offered effective, safe handling for Japanese prostate cancer with short-term follow-up. Our event suggest higher prescribed doses are related to higher toxicity rates.
Introduction
Ultrahypofractionated (UHF) radiotherapy is divers equally > 5 Gy per fraction and has gradually been recognized as a standard treatment for localized prostate cancer. The biological features of prostate cancer with a low α/β ratio have encouraged widespread adoption of UHF radiotherapy around the world. In addition to these biological advantages, UHF radiotherapy offers benefits in terms of cost effectiveness and patient convenience. In addition, patients treated with UHF radiotherapy reported significantly "less regret" and "less toxicity" than expected compared to patients treated with other radiotherapy techniques1.
The current guidelines conditionally recommend UHF radiotherapy merely for low- or intermediate-hazard patients2. However, every bit the potential advantages of UHF radiotherapy over other handling techniques are gradually confirmed, candidates for this treatment are expected to expand to not only low- and intermediate-gamble patients, just besides high-risk patients.
Meanwhile, the current state of UHF radiotherapy in Japan is unclear, although several Japanese institutions are likely to accept already started UHF radiotherapy. In improver, treatment results for Japanese patients have not withal been reported except in a few papers3,4,v. We therefore conducted a survey of the current status of UHF radiotherapy in Japan and undertook a multi-institutional retrospective assay of UHF radiotherapy for Japanese prostate cancer patients. We report herein the outcomes and chance factors of UHF radiotherapy in Japanese prostate cancer patients.
Materials and methods
Patients and treatments
We sent a questionnaire survey to effectually 160 Japanese institutions that were participants in the Japanese Radiation Oncology Study Group (JROSG) or that were equipped with CyberKnife systems between Dec 2019 and Feb 2020. The results showed that at least 10 institutions in Nippon currently employ UHF radiotherapy for localized prostate cancer, and more than 1300 patients have already received handling with UHF radiotherapy. Detailed results of the questionnaire survey are shown in the Supplementary Table S1.
Half-dozen of the 10 institutions agreed to participate in farther retrospective assay of patients treated with UHF radiotherapy. The Kitasato University Hospital institutional review lath, Shonan Fujisawa Tokusyukai Hospital institutional review board, Toyama CyberKnife Middle institutional review board, Saiseikai Yokohamashi Tobu Infirmary institutional review lath, Tobata Kyoritsu Hospital institutional review board, and National Cancer Center Hospital institutional review board approved the written report protocol. Informed consent was obtained in the class of opt-out on the web-site. Those who rejected were excluded. This study was conducted in accordance with the Declaration of Helsinki.
Table 1 shows the background characteristics of patients. More than one-half of the patients (61%) were categorized as intermediate risk based on the National Comprehensive Cancer Network criteria. More than half of the patients (57%) had received neoadjuvant androgen impecuniousness therapy (ADT), and 32% had received adjuvant ADT. The mean (± standard deviation) duration of therapy was 6.5 (± 6.3) months for neoadjuvant ADT, and 12.5 (± 12.half dozen) months for adjuvant ADT. The total dose was 35–36 Gy in 4–five fractions, prescribed as sequential or alternate-day doses. The mean biologically effective dose (BED) based on α/β = 1.5 was 222.8 Gy. Most patients in our written report population had no hydrogel spacer. Tabular array 2 shows treatment protocols in the 6 participating institutions. Four of 6 institutions used CyberKnife. Five of 6 institutions used fiducial markers implanted in the prostate.
Statistical analysis
Statistical analyses were performed using R version 3.5.i software (R Projection for Statistical Computing, Vienna, Austria). Overall survival was calculated using the Kaplan–Meier method. Biochemical failure was defined according to the Phoenix ASTRO consensus (Nadir + ii)6. Genitourinary (GU) and gastrointestinal (GI) toxicities were assessed using the National Cancer Establish Common Toxicity Criteria version iv.
A competing risk assay (Gray'due south exam and Fine and Gray'southward regression) was used for biochemical control, local recurrence, pelvic lymph-node recurrence, distant metastasis, castration-resistant prostate cancer (CRPC) and cumulative GU and GI toxicity rates. Rough rates of GU and GI toxicities are also reported for comparison with other reports. Age, T stage, Gleason score, initial prostate-specific antigen (PSA), BED, neoadjuvant ADT, and adjuvant ADT were included as variates in univariate analyses. Multivariate models for each endpoint were constructed past including all factors with values of p < 0.xx from univariate analyses. Values of p < 0.05 were considered statistically meaning.
Ideals approval and consent to participate
This written report was approved by the local institutional review boards.
Consent for publication
Informed consent was obtained in the class of opt-out on the web-site. Those who rejected were excluded.
Results
The median elapsing of follow-up was 32 months (range 22–97 months). Two- and 3-year overall survival rates were 99.half-dozen% (95% CI 0.99–100%) and 99.1% (95% CI 97.nine–100%), respectively. No prostate cancer deaths were reported. Two- and 3-twelvemonth biochemical control rates were 97.7% (95% CI 95.2–99.0%) and 96.4% (95% CI 93.3–98.4), respectively. Corresponding rates of each chance category were as follows: low risk, 100% and 100% (95% CI na); intermediate risk, 97.five% (95% CI 94.0–99.two%) and 96.6% (95% CI 92.vii–98.seven%); and loftier run a risk, 97.1% (95% CI ninety.viii–99.5%) and 93.seven% (95% CI 83.two–98.6%), respectively. Initial PSA and neoadjuvant ADT were detected every bit take a chance factors for biochemical recurrence by multivariate analysis (Tabular array iii, Fig. i).
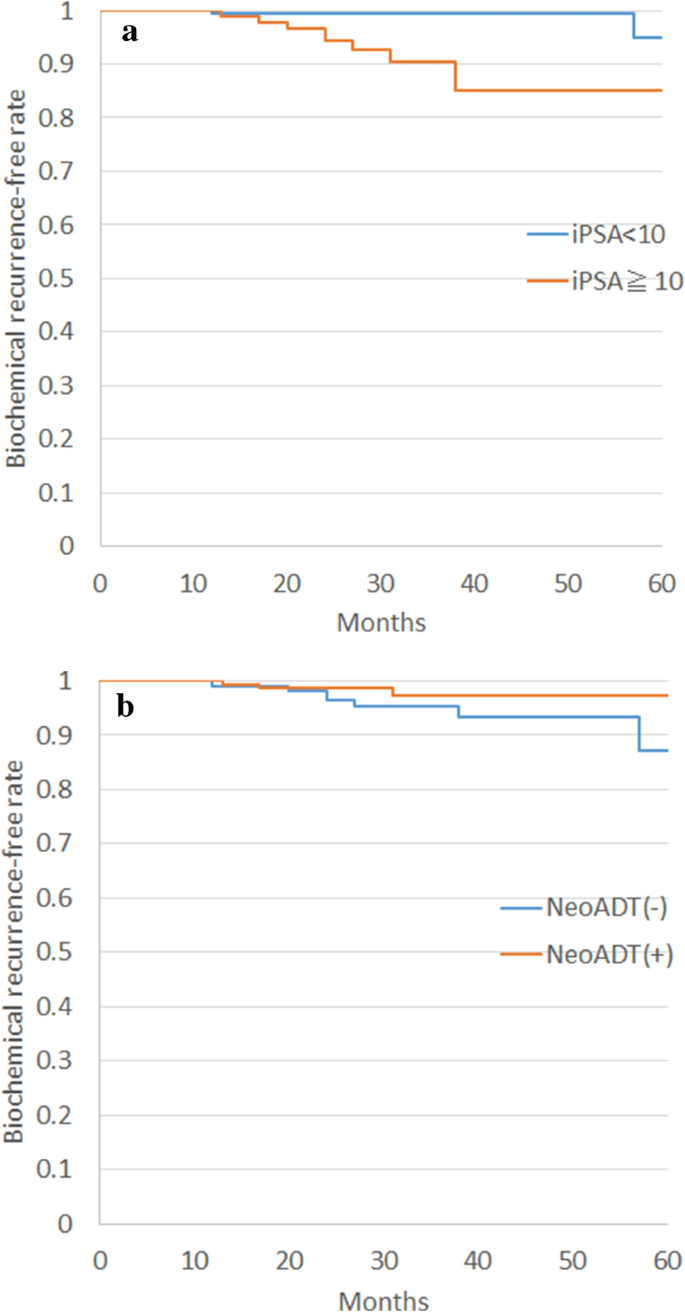
Differences in biochemical recurrence-free rates depend on initial PSA value (a) and neoadjuvant androgen deprivation therapy (b).
Two-year local recurrence, pelvic lymph-node recurrence, and afar metastasis rates were 0.four%, 0.iv%, and 1.ii%, respectively. Corresponding rates at three years were 1.3%, 0.four%, and one.8%, respectively. Ii- and three-yr CRPC rates were 0.4% and i%, respectively.
Table four shows crude rates of astute and tardily toxicities. Grade 3 toxicity rate was lower than 1%. No class 4 or college toxicity was seen during follow-up. The almost common acute ≥ Grade ii GU toxicities were frequency (17/37, 46%), retention (thirteen/37, 35%), and pain (3/37, 4%). Corresponding GI toxicities were diarrhea (7/13, 54%), proctitis (3/13, 23%), and bleeding (iii/13, 23%). The most common late > Course 2 GU toxicities were frequency (8/xviii, 44%), retention (2/18, 11%), and pain (ii/eighteen, 11%). The corresponding GI toxicity was bleeding (ten/12, 83%).
2- and 3-year cumulative ≥ Form two late GU toxicity rates were 5.8% and 7.iv%, respectively. Corresponding rates of GI toxicities were iii.nine% and 4.5%, respectively. BED was detected equally a adventure cistron for ≥ Grade 2 tardily GU and GI toxicities (Table v, Fig. 2). No other variables was detected with values of p < 0.xx in univariate analyses.
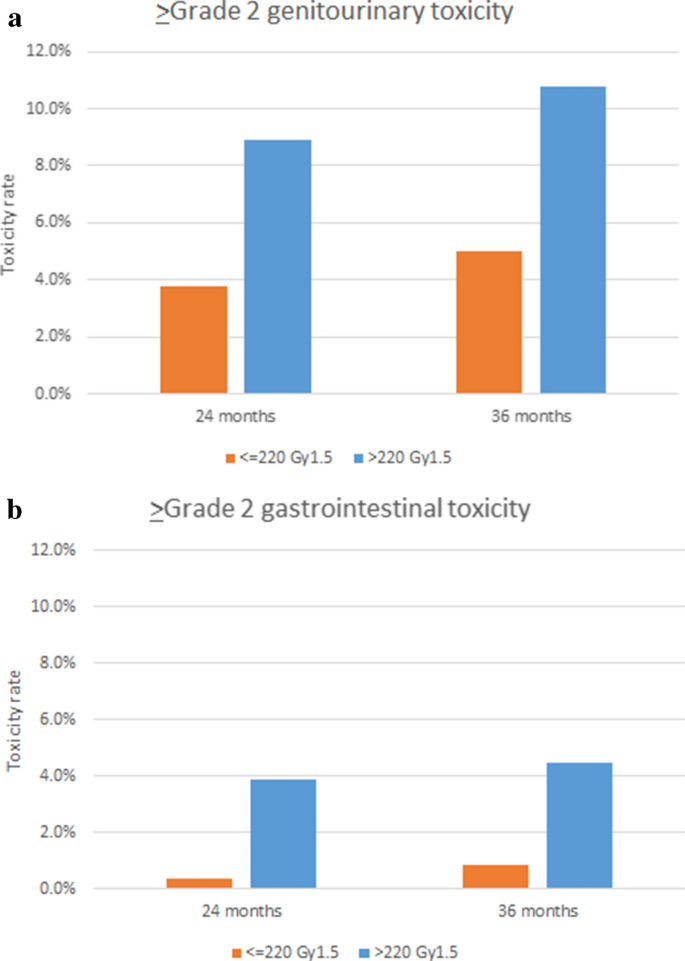
Crude toxicity rates for Course ≥ 2 genitourinary (a) and gastrointestinal, (b) toxicities depend on biologically equivalent doses (> 220 or ≤ 220 Gyane.5).
Discussion
Our retrospective assay showed that the UHF radiotherapy is safe and effective for Japanese patients, at to the lowest degree in a short-term follow-up. Considering recently reported long-term7 and big cohort8 outcomes from Western countries, this treatment is likewise valuable every bit a handling option for Japanese prostate cancer patients. Although a cautious approach is warranted until long-term results go available, there are no obstacles to applying UHF radiotherapy for Japanese patients.
Our analysis revealed that prescription doses were significantly related to toxicity rates. The American Society for Radiations Oncology (ASTRO), American Society of Clinical Oncology (ASCO), and American Urological Association (AUA) guidelines recommend prescription doses betwixt 35 Gy and 36.25 Gy in 5 fractions, and doses above 36.25 Gy are not suggested outside the setting of clinical trials due to the risk of late toxicities2. Tabular array 6 shows the results of previous reports of relatively loftier incidences of ≥ Grade 2 toxicitiesvii,nine,ten,11,12,thirteen,14,15,16. Although information technology was not always high-dose prescriptions that led to high toxicity rates in previous studies, prescribed doses seem related to more than severe toxicity, as our study suggested. Considering our study included patients who participated in dose-escalation trials testing relatively high doses, these patients suffered relatively severe late toxicities compared to patients treated with current standard doses following the guidelines4.
Koontz et al. published a review analyzing data from pioneering institutions and reported that over 100 Gy-equivalents in 2-Gy fractions might cause higher rates of > Form two toxicities17. Considering the schedule of 36 Gy/4 fractions included in our study was equal to 108 Gy-equivalents in 2-Gy fractions and a BED of 226 Gy, our results appear consistent with their suggestion.
From the perspective of toxicity, a lower prescribed dose might be suitable, especially for depression- and favorable intermediate-run a risk patients. However, a lower dose might cause lower tumor command, and the importance of dose escalation is well known in conventional fractionationeighteen,19. In the field of UHF radiotherapy, Zelefsky et al. suggested the importance of dose escalation. Although long-term control rates were not adamant, they reported positive biopsy rates of 47.6%, nineteen.two%, sixteen.vii% and seven.7% later 32.five Gy, 35 Gy, 37.five Gy and 40 Gy in v fractions, respectively20. These results suggest that unfavorable tumor control rates might exist seen in lower-dose groups.
Helou et al. reported that the 3-year PSA level correlated with the prescribed dose in a comparison between xl Gy (0.27 ng/ml) and 35 Gy (0.64 ng/ml) in five fractions. The higher dose of xl Gy was an independent predictor of a lower 3-year PSA level in their multivariate analysis16. The 3-twelvemonth PSA value was previously establish to offer an early predictor of biochemical failure after high-dose-charge per unit brachytherapy21. The concept of a well-balanced, optimal dose during UHF radiotherapy thus remains contentious and should exist explored in future trials.
Meanwhile, periprostatic hydrogel spacers have been approved for use with transperineal injection in Japan since 2017. Current practices in UHF radiotherapy are thus expected to commonly exist combined with spacer injection, and may subtract astringent toxicities even using the same dose levels22.
Regarding risk factors for biochemical recurrence, our results were not surprising from the point of view of feel with conventional external beam radiotherapy (EBRT). The nomogram established by Kattan et al. more 20 years ago revealed that the initial PSA level was the well-nigh powerful indicator of biochemical recurrence23. ADT is well known as some other powerful indicator of biochemical recurrence24. Our results thus suggested that UHF radiotherapy shows similar trends to conventional EBRT, although our curt follow-up makes the value of these take a chance factors difficult to ostend.
Because our study was a retrospective analysis, several limitations should be considered. First, equally collected items were limited, other factors might correlate with tumor control and toxicity rates. 2d, our analysis of toxicities might take been biased due to the lack of information on dose-book histograms. Third, because various treatment schedules were used at each infirmary, results reported in this newspaper would have varied depending on treatment schedules. Fourth, because no follow-upwardly schedule was divers, the timings of follow-upward visits and examinations among hospitals were highly heterogeneous. Tumor control and toxicity rates might thus be relatively cryptic.
Determination
This multi-institutional analysis showed that UHF radiotherapy is effective for Japanese prostate cancer with limited severe toxicity. However, the optimal dose during UHF radiotherapy should be continuously explored, as our results also suggested that higher prescribed doses were related to higher toxicity rates.
Information availability
The datasets used and/or analysed during the electric current study are available from the respective writer on reasonable request.
Abbreviations
- UHF:
-
Ultrahypofractionated
- GU:
-
Genitourinary
- GI:
-
Gastrointestinal
- ASTRO:
-
American Gild for Radiation Oncology
- JROSG:
-
Japanese Radiations Oncology Report Group
- ADT:
-
Androgen deprivation therapy
- CRPC:
-
Castration-resistant prostate cancer
- PSA:
-
Prostate-specific antigen
- BED:
-
Biologically effective dose
- ASCO:
-
American Society of Clinical Oncology
- AUA:
-
American Urological Association
- EBRT:
-
External beam radiotherapy
References
-
Shaverdian, N. et al. Exploring value from the patient'due south perspective between mod radiation therapy modalities for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 97(3), 516–525 (2017).
-
Morgan, S. C. et al. Hypofractionated radiation therapy for localized prostate cancer: An ASTRO, ASCO, and AUA evidence-based guideline. J. Clin. Oncol. https://doi.org/ten.1016/j.juro.2018.10.001 (2018).
-
Nakamura, R. et al. Stereotactic torso radiotherapy with a single isocentre for multiple pulmonary metastases. BJR Case Rep. 6(4), 20190121 (2020).
-
Kainuma, T. et al. A phase I dose-escalation trial of stereotactic body radiotherapy using 4 fractions for patients with localized prostate cancer. Radiat. Oncol. 14(1), 158 (2019).
-
Kobayashi, H. et al. Distribution assay of hydrogel spacer and evaluation of rectal dose reduction in Japanese prostate cancer patients undergoing stereotactic body radiation therapy. Int. J. Clin. Oncol. 26, 736 (2021).
-
Roach, M. et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Briefing. Int. J. Radiat. Oncol. Biol. Phys. 65(4), 965–974 (2006).
-
Katz, A. Stereotactic trunk radiotherapy for low-chance prostate cancer: A ten-twelvemonth analysis. Cureus. 9(nine), e1668 (2017).
-
Kishan, A. U. et al. Long-term outcomes of stereotactic trunk radiotherapy for low-risk and intermediate-chance prostate cancer. JAMA Netw. Open up. 2(2), e188006 (2019).
-
Aluwini, South. et al. P068 stereotactic torso radiotherapy with 4 fractions for low-and intermediate-run a risk prostate cancer: Acute and late toxicity. Eur. Urol. Suppl. 12(6), 156 (2013).
-
Chen, L. N. et al. Stereotactic body radiations therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat. Oncol. 8, 58 (2013).
-
Meier, R. M. et al. Multicenter trial of stereotactic body radiation therapy for depression-and intermediate-chance prostate cancer: Survival and toxicity endpoints. Int. J. Radiat. Oncol. Biol. Phys. 102(2), 296–303 (2018).
-
Zimmermann, M. et al. Prospective stage Two trial of once-weekly hypofractionated radiation therapy for low-gamble adenocarcinoma of the prostate: Tardily toxicities and outcomes. Clin. Oncol. 28(six), 386–392 (2016).
-
Kim, D. W. et al. Predictors of rectal tolerance observed in a dose-escalated phase one–2 trial of stereotactic trunk radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 89(3), 509–517 (2014).
-
Bernetich, M. et al. SBRT for the primary handling of localized prostate cancer: The effect of gleason score, dose and heterogeneity of intermediate risk on outcome utilizing two.2014 NCCN risk stratification guidelines. Front. Oncol. iv, 312 (2014).
-
Zhang, 50. et al. Receiver operating curves and dose-book analysis of late toxicity with stereotactic body radiations therapy for prostate cancer. Pract. Radiat. Oncol. 7(two), e109–e116 (2017).
-
Helou, J. et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate gamble prostate cancer: Is at that place an optimal dose?. Radiother. Oncol. 123(3), 478–482 (2017).
-
Koontz, B. F., Bossi, A., Cozzarini, C., Wiegel, T. & D'Amico, A. A systematic review of hypofractionation for primary management of prostate cancer. Eur. Urol. 68(iv), 683–691 (2015).
-
Viani, Grand. A., Stefano, Due east. J. & Afonso, South. L. Higher-than-conventional radiations doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 74(5), 1405–1418 (2009).
-
Zelefsky, 1000. J. et al. Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int. J. Radiat. Oncol. Biol. Phys. 71(4), 1028–1033 (2008).
-
Zelefsky, M. J. et al. 5-year outcomes of a phase I dose escalation study using stereotactic trunk radiosurgery for patients with low and intermediate take a chance prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 104, 42 (2019).
-
Helou, J. et al. High dose-rate brachytherapy boost for intermediate gamble prostate cancer: Long-term outcomes of 2 different treatment schedules and early biochemical predictors of success. Radiother. Oncol. 115(1), 84–89 (2015).
-
Karsh, L. I. et al. Absorbable hydrogel spacer utilise in prostate radiotherapy: A comprehensive review of phase 3 clinical trial published data. Urology 115, 39–44 (2018).
-
Kattan, M. W. et al. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J. Clin. Oncol. eighteen(19), 3352–3359 (2000).
-
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase Three randomised trial. Lancet 360(9327), 103–106 (2002).
Acknowledgements
This study was supported in part by the Japanese Radiations Oncology Written report Grouping.
Funding
This report was supported in part by the Japanese Radiation Oncology Written report Group.
Author information
Affiliations
Contributions
H.I., H.T. and H.K. drafted the manuscript. H.I. and 1000.Due north. participated in the pattern of the study. H.I. performed the statistical analysis. K.N. supervised the study. H.I., H.Northward., Thousand.West., E.G., 1000.T., H.K., T.East., H.I. and 1000.I. collected medical data near this report. All authors read and canonical the concluding manuscript.
Respective author
Ethics declarations
Competing interests
Dr. Ishiyama reports grants from Nihon Medi-physics, Co,Ltd., outside the submitted work. Dr. Inaba reports grants from Elekta Thousand.K., exterior the submitted work.
Boosted information
Publisher'south note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Data
Rights and permissions
Open Access This commodity is licensed under a Creative Commons Attribution four.0 International License, which permits utilize, sharing, adaptation, distribution and reproduction in whatsoever medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables licence, and point if changes were made. The images or other third party material in this article are included in the commodity'southward Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the commodity's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted employ, you volition demand to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this commodity
Cite this commodity
Ishiyama, H., Tsumura, H., Nagano, H. et al. Multi-institutional retrospective analysis of ultrahypofractionated radiotherapy for Japanese prostate cancer patients. Sci Rep 11, 13194 (2021). https://doi.org/10.1038/s41598-021-92307-8
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-021-92307-8
Further reading
Comments
Past submitting a comment you hold to abide by our Terms and Community Guidelines. If yous notice something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-021-92307-8